Abstract
BACKGROUND: Novel agents added to front-line treatments for adult ALL yield high rates of measurable residual disease (MRD) negativity and survival. However, these approaches are infeasible outside large centers and in lower-resource settings. HyperCVAD is commonly used regardless of Philadelphia chromosome (Ph) status but has limitations, particularly in older patients (pts). Because of its efficacy for high-grade lymphomas (NEJM 2013, p. 1915), we hypothesized that DA-EPOCH would be active and safe in adult ALL.
METHODS: Details of this study have been reported (ASH 2018, #1519; NCT03023046). Adults with newly-diagnosed ALL were eligible if not candidates for pediatric-inspired therapy (ie, Ph+, age ≥ 40). DA-EPOCH was given with G-CSF (Blood 2002, p. 2685). After treatment of the 1 st 5 pts, the dose-adjustment paradigm was employed once cytopenias were not due to ALL, typically after cycle 2. If Ph+, imatinib (IM) 600 mg or dasatinib (DAS) 100 mg daily on Days 1-14 of each cycle was added. If CD20+, rituximab 375 mg/m 2 was given once per cycle regardless of Ph status. Up to 8 cycles could be given followed by maintenance (POMP ± TKI) or allogeneic transplant (HCT). Pts with WBC > 30K/µL or LDH > 3x upper limit of normal at diagnosis received 10 doses of intrathecal methotrexate as CNS prophylaxis, and others received 8.
Response was determined by bone marrow aspirate morphology (morph), multiparameter flow cytometry (MFC) and (for Ph+) BCR-ABL RT-PCR, with complete molecular response (CMR) assigned when the latter was undetectable. When MRD- by MFC (MFC-) per EuroFlow criteria (Leukemia 2010, p. 521), high-throughput sequencing-based MRD testing (HTS; ClonoSEQ) was performed. IKZF1plus was identified by chromosomal microarray as defined previously (JCO 2018, p. 1240).
The primary endpoint was rate of MFC- remission within 4 cycles of therapy. Based on an institutional historical rate of MFC- after ≤ 4 cycles of 50% with hyperCVAD + TKI for Ph+ ALL (Am J Hematol 2018, p. 546), we defined success if we observed such remissions in ≥ 70%. A Simon 2-stage design with α = 0.09 and 80% power led to a sample size of 28 pts and success if ≥ 18 were MFC- after ≤ 4 cycles. For Ph-, we enrolled in cohorts (up to 25 total pts) if the upper bound of a 90% confidence interval (CI) stayed ≥ 59%, our historical rate with hyperCVAD. Follow-up provided to 7/1/21.
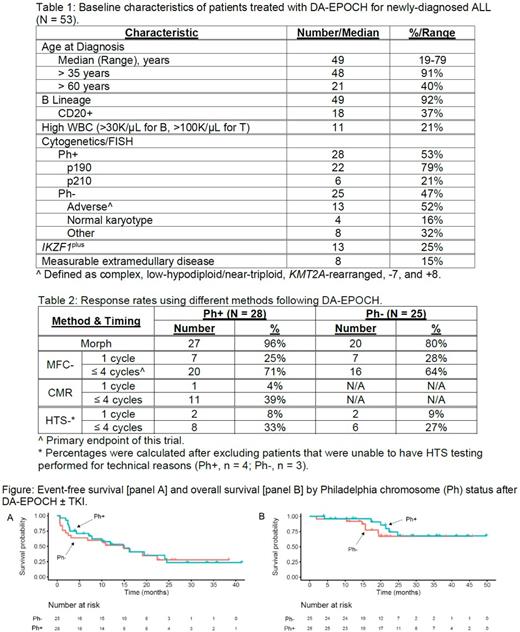
RESULTS: 53/54 enrolled pts were evaluable. Baseline characteristics are in Table 1 and response rates in Table 2. For Ph+ ALL, 20 of 28 pts (71%; 90% CI = 54-85%) reached MFC- after ≤ 4 cycles; TKI used was DAS for 23 pts and IM for 5 pts. In Ph- ALL, 16 of 25 pts (64%; 90% CI = 46-80%) were MFC- after ≤ 4 cycles.
Median follow-up of survivors was 22 months (mo; range: 4-50 mo). Median and 2-yr EFS were 15 mo and 32% (respectively); median OS was not reached, and 2-yr OS was 70% (Figure). In those age > 60 yrs, 2-yr EFS was 49% and OS was 71%. In univariate Cox models, neither WBC, age, adverse-risk cytogenetics, IKZF1plus, nor MFC- after ≤ 4 cycles were significantly associated with EFS or OS; the strongest was high-risk WBC with EFS (hazard ratio 2.1, p = 0.068).
In sum, 36 pts reached MFC-: relapse occurred in 12 of 23 pts (52%) given maintenance therapy vs 4 of 12 pts (33%) who received HCT (p = 0.48 by Fisher exact test). Of 18 pts without MRD by HTS, 9 (50%) relapsed. None of the 6 pts given blinatumomab for MRD after DA-EPOCH have relapsed. Four pts (8%) had isolated CNS relapse.
44 pts (83%) developed ≥ 1 Grade (Gr) 3+ non-hematologic (heme) AE related to DA-EPOCH, with a mean of 2 per pt. Those seen in ≥ 10% of pts were febrile neutropenia/infection (23 Gr 3, 2 Gr 4); mucositis (7 Gr 3); and ALT increase (6 Gr 3). There was only 1 treatment-related death (2%; gastric hemorrhage). Twelve pts (23%) discontinued due to acute or cumulative toxicity. As for heme AEs in pts treated after cycle 1 (ie, when ALL was not the cause), only 15 pts (34%) had a Gr 4 platelet count at any time.
CONCLUSIONS: DA-EPOCH yields comparable response and survival rates (with manageable toxicity) as more logistically-complex therapies in primarily high-risk ALL. When TKI was added for Ph+ disease, rates of MFC- exceeded the predefined threshold for success. Its efficacy and safety profile make it an appealing backbone to which novel agents may be added. Pending results of ongoing multivariable analyses comparing DA-EPOCH to historical treatments, DA-EPOCH could be considered a standard option, particularly in older adults and/or lower-resource settings.
Cassaday: Pfizer: Consultancy, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Merck: Research Funding; Vanda Pharmaceuticals: Research Funding. Percival: Abbvie: Research Funding; Nohla Therapeutics: Research Funding; Cardiff Oncology: Research Funding; Glycomimetics: Research Funding; Biosight: Research Funding; BMS/Celgene: Research Funding; Pfizer: Research Funding; Oscotec: Research Funding; Trillium: Research Funding. Halpern: Gilead: Research Funding; Abbvie: Consultancy; Agios Pharmaceuticals: Research Funding; Tolero Pharmaceuticals: Research Funding; Novartis: Research Funding; Bayer: Research Funding; Imago Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Agios: Consultancy; Nohla Therapeutics: Research Funding; Pfizer: Research Funding. Becker: Glycomimetics: Research Funding; CVS Caremark: Consultancy; Abbie: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Cardiff Oncology: Research Funding; SecuraBio: Research Funding. Oehler: BMS: Consultancy; OncLive: Honoraria; Takeda: Consultancy; Pfizer: Research Funding. Shustov: Seagen Inc.: Research Funding. Orozco: Actinium Pharmaceuticals, Inc.: Other: site PI for clinical trial(s) sponsored by Actinium, Research Funding. Walter: BMS: Consultancy; Astellas: Consultancy; Agios: Consultancy; Amphivena: Consultancy, Other: ownership interests; Selvita: Research Funding; Pfizer: Consultancy, Research Funding; Jazz: Research Funding; Macrogenics: Consultancy, Research Funding; Immunogen: Research Funding; Celgene: Consultancy, Research Funding; Genentech: Consultancy; Janssen: Consultancy; Kite: Consultancy; Aptevo: Consultancy, Research Funding; Amgen: Research Funding. Radich: Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees.
Rituximab is not FDA approved for the treatment of acute lymphoblastic leukemia.